Fluxnano
FLUXNANO – MANAGEMENT OF BIOLOGICAL TEST DATA IN A NANOTECHNOLOGY LABORATORY – COMPLEMENTARY MATERIAL
ABSTRACT
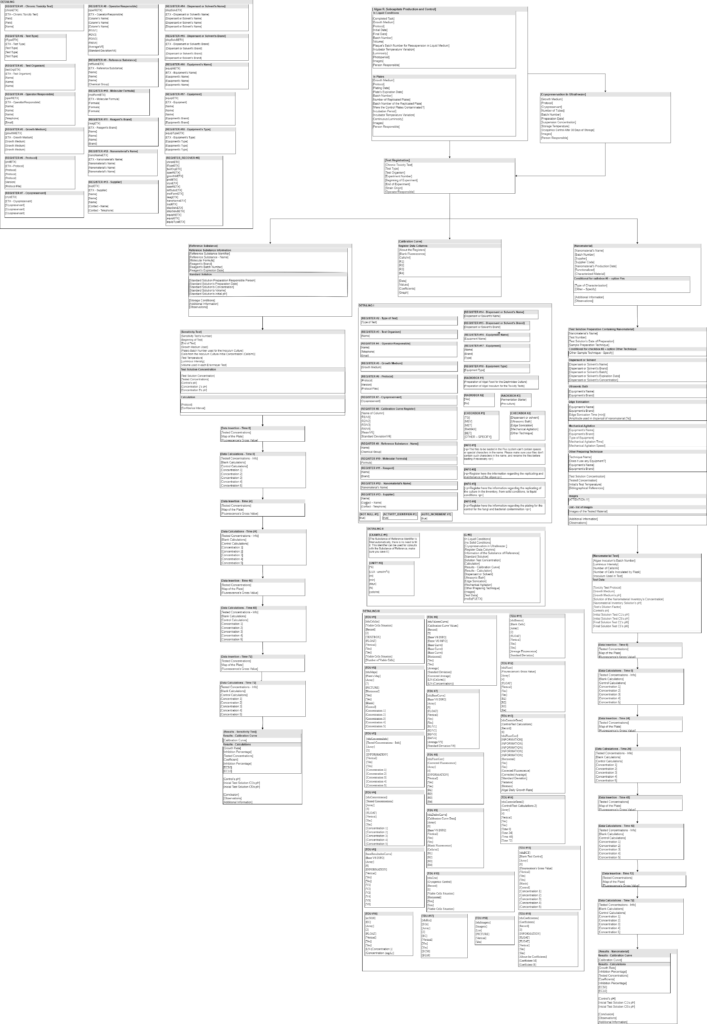
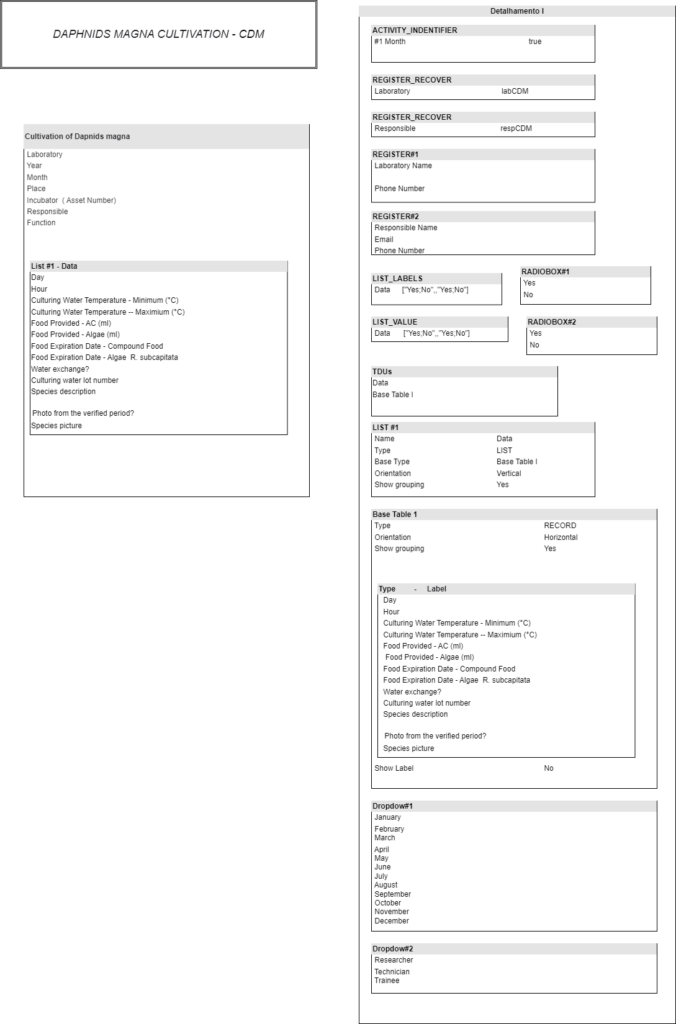
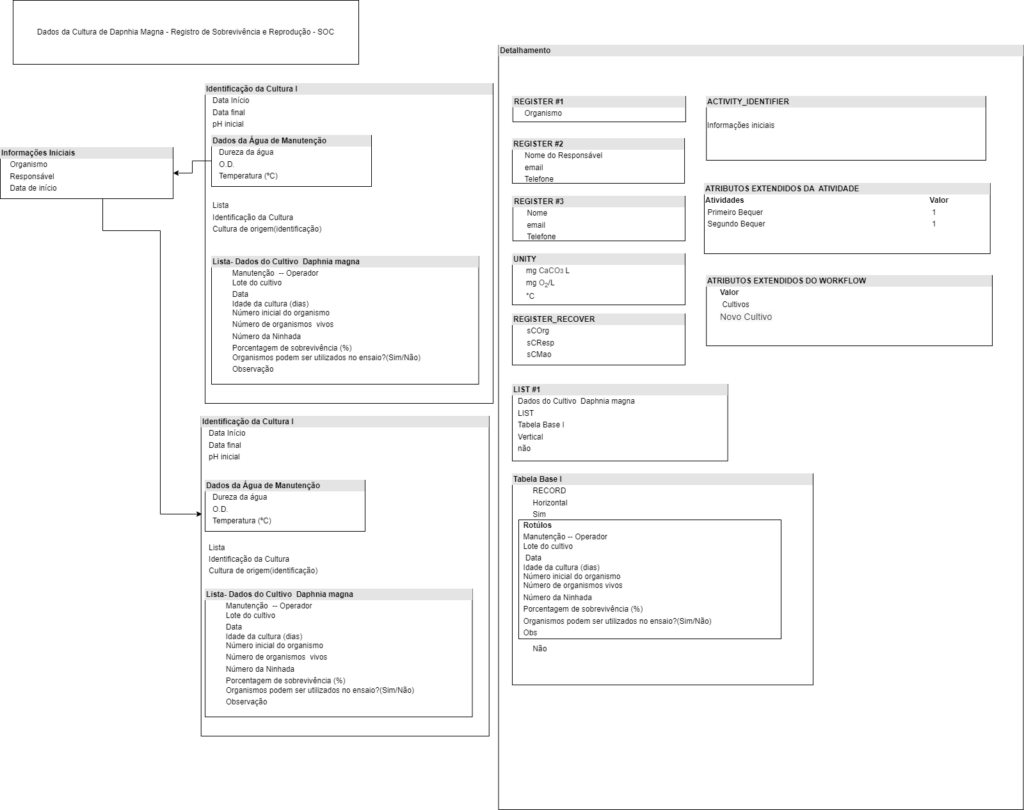
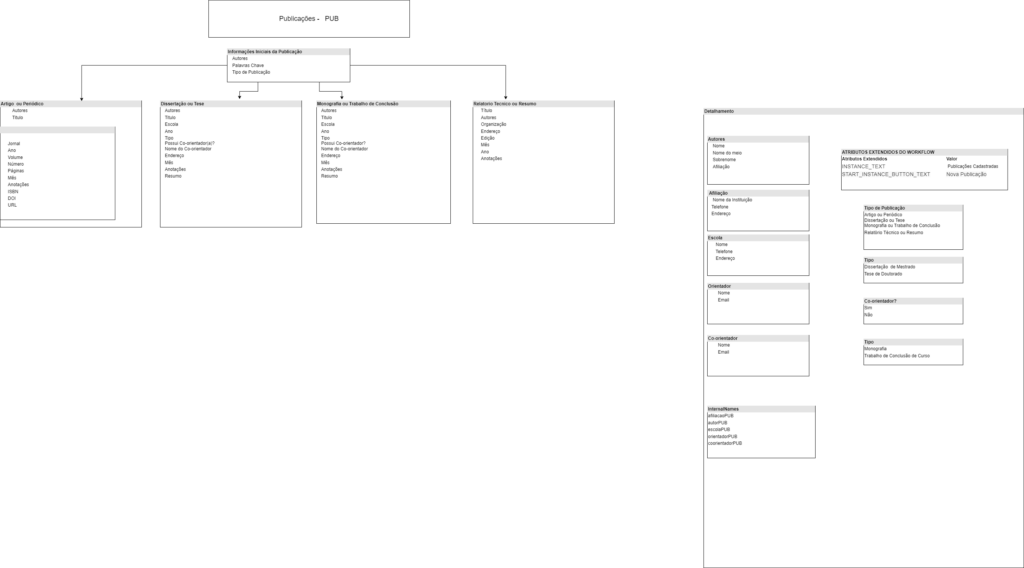
Laboratory data management is a critical process that becomes more complex every day. To facilitate this process a system to obtain and treat this information is needed. The volume of information generated by a modern lab is immense, and therefore it is necessary to organize this information and all laboratory processes. One type of tool to accomplish this task are LIMS, or Laboratory Information Management Systems. In this work we will model and implement the workflow of the Laboratório de Saúde Meio Ambiente e Segurança do CTNANO/UFMG using a workflow based LIMS. This work contributes to the understanding of the relation between LIMS, laboratory workflows and biological data management. It also provides important information to research institutions interested in improving their lab data management. It presents an opportunity to explore the advantages and limitations of using a LIMS in a real research context.
Keywords: LIMS, laboratory data management, nanotechnology
IMAGES